
|
Introduction
|
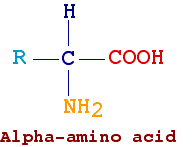
Amino acids are molecules carrying both a carboxyl (-COOH) and an amine (-NH2) group. Here follows some example:
 |
 |
In the first example, the two functional groups are bound to the same carbon atom (a-amino acid) while in the second example the two groups are bound to two different carbon atoms (gamma-aminoacids)
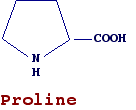
Amino acids found in proteins are a-amino acids, i.e., they have amino and carboxyl groups bound to the same carbon atom. An exception is proline, which differs from other amino acids in having a secondary amino group. Furthermore its side chain bounds both the amino and carboxylic group forming a pyrrolidinic ring, it is generally referred as a-imino-acid (a very obsolescent term).
Amino acids differ on the nature of R (also indicated as side chain). Only 20 amino acids (see table below) are commonly found in proteins. With the exception of glycine (R = H), the alpha-carbon of all amino acids is asymmetric (i.e., it binds four different groups). Therefore, these amino acids are optically active and they can exist in a form L and in a form D. Amino acids found in proteins have the L form.
|
Amino acid
|
Three / One letter code
|
Amino acid
|
Three / One letter code
|
|
|
|
||
|
|
|
Leu / L
|
|
|
|
|
Lys / K
|
|
|
|
|
Met / M
|
|
|
|
|
Phe / F
|
|
|
|
|
Pro / P
|
|
|
|
|
Ser / S
|
|
|
|
|
Thr / T
|
|
|
|
|
Trp / W
|
|
|
|
|
Tyr / Y
|
|
|
|
|
Val / V
|
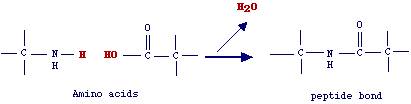 Since
in proteins a-NH2 and a-COOH
groups are involved in amide (peptide) bonds, the classification of amino acids
is based on the nature of the group R (side chain) which determines the properties
of a protein. On the basis of the physico chemical properties of their side
chains, amino acid can be grouped in six different groups which well correspond
to those amino acids having high probability to interchange one other during
evolution. The letters indicating the six different groups (S, H, N, F, C, V;
see below) are referred to as the MDM (Mutation Data Matrix) alphabet.
Since
in proteins a-NH2 and a-COOH
groups are involved in amide (peptide) bonds, the classification of amino acids
is based on the nature of the group R (side chain) which determines the properties
of a protein. On the basis of the physico chemical properties of their side
chains, amino acid can be grouped in six different groups which well correspond
to those amino acids having high probability to interchange one other during
evolution. The letters indicating the six different groups (S, H, N, F, C, V;
see below) are referred to as the MDM (Mutation Data Matrix) alphabet.
The Mutation Data Matrix is the result of the comparison of closely related amino acid sequences. From this comparison, Dayhoff (1979) observed that only some amino acid replacements are evolutionary acceptable. For example, tryptophan is more frequently replaced by another aromatic amino acid (phenylalanine and tyrosine) rather than by any another amino acid (see sequences comparison).
This group comprises glycine, alanine, proline and the two hydroxyacids
threonine and serine. They are slightly hydrophylics
but do not have ionizable groups at neutral pH, thus they do not contribute
to the net charge of proteins.
|
(abbreviations) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Also classified as an hydroxyamino acid, it has two
asymmetric carbon atoms.
|
|
|
|
|
Also classified as an hydroxyamino acid.
|
|
|
|
|
This group comprises amino acids with aliphatic side chain: valine,
leucine, isoleucine and the sulphur-containing methionine. These
amino acids do not contribute to the net charge of proteins (at neutral pH).
|
(abbreviations) |
|
|
|
|
(Val or V) |
|
|
|
|
(Leu or L) |
|
|
|
|
(Ile or I) |
|
|
|
|
(Met or M) |
|
|
This group includes amino acids having aromatic rings in the side chain:
phenhylalanine, tyrosine and tryptophan. Side chains in this group
do not have ionizable groups at neutral pH, thus they do not contribute to the
net charge of proteins.
|
(abbreviations) |
|
|
|
|
(Phe or F) |
|
|
|
|
(Tyr or Y) |
|
|
|
|
(Trp or W) |
|
|
This group includes amino acids having an additional carboxyl groups in the side chain (aspartic acid and glutamic acid) and their amides (asparagine and glutamine).
Acids are negatively charged at neutral pH thus contributing to the net charge of proteins.
|
|
|
|
|
|
(Asp or D) |
|
|
|
|
(Glu or E) |
|
|
|
|
(Asn or N) |
|
|
|
|
(Gln or Q) |
|
|
This group includes amino acids having additional amino groups in the side chain (lysine, arginine and histidine).
They are positively charged at neutral pH thus contributing to the net charge of proteins.
|
(abbreviations) |
|
|
|
|
(Lys or K) |
|
|
|
|
(Arg or R) |
|
|
pKa = 12.48 |
|
(His or H) |
|
|
pKa = 6 |
This group includes only cysteine, having an SH group (sulphydril group) in the side chain.
Cysteine can occur as cystine in which two molecules of cysteines are linked by a disulfide bond.
SH group is not charged at neutral pH thus this amino acid do not contribute to the net charge of proteins.
|
(abbreviations) |
|
|
|
|
|
|
|
|
|
|
|
|
|