In this section we shall deal with solutions containing a mixture of an acid (AH) and a base (BOH). In such solutions reactions 1-5 take place
| 1. Acid dissociation | 2. Base dissociation | 3. Neutralization |
| AH + H2O |
BOH + H2O |
H+ + OH- --> H2O |
| 4. Hydrolysis of A- | 5. Hydrolysis of B- |
| A- + H2O |
B- + H2O |
It follows that the pH of a solution containing an acid and a base depends on the respective amount of the two components (neutralization reaction) and on their ability to react with water (hydrolysis).
| However, if nAH and nBOH are the moles of the acid and the base respectively, it always results that pH < 7 for nAH > nBOH and pH > 7 for nAH < nBOH. |
![]() The most simple case:
reaction of a strong acid with a strong base.
The most simple case:
reaction of a strong acid with a strong base.
In this case neither B+ or A- (arising from the full dissociation of the acid and the base respectively) can react with water, thus only the reaction of neutralization has to be considered.
Here follows the possible cases:
| For nAH = nBOH, pH = 7 | In fact all H+ and OH- are transformed in water through the neutralization reaction. |
| For nAH > nBOH, pH < 7 |
Only the excess of the acid (nAH - nBOH) is to be considered |
| nAH < nBOH, pH > 7 | Only the excess of the base (nBOH-nAH) is to be considered |
![]() A more involved case: reaction of a weak acid with a strong base (or vice
versa).
A more involved case: reaction of a weak acid with a strong base (or vice
versa).
All the examples reported here, refers to a weak acid and a strong base. However, the reader should realize that problems involving a weak base and a strong acid are perfectly equivalents.
In a solution containing a weak acid and a strong base only the reactions 1-4 should be considered in fact B- (arising from a strong base) can not hydrolyze.
| 1. Acid dissociation | 2. Base dissociation | 3. Neutralization |
| AH + H2O |
BOH + H2O |
H+ + OH- --> H2O |
| 4. Hydrolysis of A- | |
| A- + H2O |
In principle, in order to calculate the pH of a such solution we should consider all the equilibria, but we will see that reasonable approximations can be made to solve the problem. Let's consider the differents cases:
![]() [base] >
[acid]
[base] >
[acid]
In this case the acid will be full converted in its conjugate base (neutralization reaction, 3), thus we should consider only the reaction of hydrolysis (4). However, the presence of an excess of OH-, arising from the full dissociation of the base, will repress the reaction of hydrolysis according to LeChatelier' s principle (the common ion effect). Therefore, we can calculate the pH by considering only the excess of the base.
The case of solution in which [base] = [acid], is perfectly equivalent to a solution of the salt formed by the acid and the base. For example, a solution containing 0.5 moles of acetic acid and 0.5 moles of NaOH in one liter of water, is perfectly equivalent to a 0.5 M solution of sodium acetate. In this case the only reaction one should consider is the hydrolysis reaction of the weak component (4). The hydrolysis constant (5)
![]() [AH]
[OH-]
[AH]
[OH-]
![]() 5.
Ki = -----------
5.
Ki = -----------
![]() [A-]
[A-]
can be simplified by assuming that
[AH] = [OH-] i.e., we neglect OH- contributed from water;
[A-] = Cs (initial concentration of the salt), i.e., we neglect the small amount of A- which hydrolizes.
Therefore, the hydrolysis constant can be written as in 6 and remembering that Ki = Kw/Ka, we obtain equation 7.
Taking -Log:
pOH = 1/2 pKw - 1/2 pKa - 1/2 Log Cs
pH = pKw - pOH = pKw - (1/2 pKw - 1/2 pKa - 1/2 Log Cs)
pH = 1/2 pKw + 1/2 pKa + 1/2 Log Cs
It should be evident that in the case of a weak base and a strong acid we should consider the hydrolysis constant of the base and follow the same reasoning made above.
Solutions containing an excess of a weak acid with respect to a strong base (or viceversa) deserves more attention for they have the property to minimize change in pH when small amounts of an acid or a base is added to them (buffers). Let' consider the case in which the acid is in excess with respect to the base (we still remember that the treatment of a solution containing a weak base in excess with rispect to a strong acid is perfectly equivalent), such solution will contain both an appreciable amount of
AH (the undissociated acid, which also arises from hydrolysis reaction)A- (the conjugated base produced by neutralization and dissociation reactions)
Therefore, in order to calculate the concentrations of AH and A- we should consider the following equilibria: dissociation, hydrolysis and neutralization. However, we can simplify the problem by making the following considerations according to LeChatelier's principle (the common ion effect):
In a weak acid solution, only a very small amount of the acid is dissociated. Furthermore, the presence of an appreciable amount of its conjugated base (produced by the base) further repress the dissociation. Thus it can be assumed, with a good approximation, that at equilibrium [A-] = the concentration of the strong base added;
In a pure solution of a conjugated base of a weak acid only a small fraction reassociate and the presence of appreciable amount of the acid further repress the hydrolysis reaction. Thus it can be assumed, with a good approximation, that at equilibrium [AH] = [AH] initial - the concentration of the strong base added.
Therefore, the pH of a solution containing an
excess of weak acid with respect to a strong base, can be calculated by
using the expression for H3O+ arising from the ionization
constant of the acid (1 and 2). Equation 2
is known as the Henderson-Hasselbach equation. The solutions for
which the Henderson-Hasselbach equation is valid are known as buffer solutions.
From Henderson-Hasselbach equation it can be deduced that:
the pH of a solution containing appreciable amount of a weak acid and its conjugated base depends on the ratio of [AH] and [A-] |
where [A-] = [base] |
|
The property of buffer solutions, to minimize change in pH when small amounts of an acid or a base are added, is due to the presence in the solution of appreciable amount of a weak acid and its conjugated base (or a weak base and its conjugated acid). Here, we will illustrate buffers mechanism by using a numerical example. As first, let's calculate the pH of a solution obtained by dissolving in 1 liter of water 0,095 moles of acetic acid (pK=4.75) and 0,05 moles of NaOH. The pH of this solution is calculated by using the H-H equation (see 1). Now, let's calculate the change in pH when 0.005 moles of HCl are added at the solution described above. Reaction 2 will take place, and thus the addition of 0.005 moles of HCl will result in an increase of acetic acid concentration of 0.005 moles and a decrease of acetate concentration of 0.005 moles. The new value of pH will be 4.70. Therefore, the addition of HCl produces a very small variation of pH (less than 0.1 unit). This variation should be compared to that occurring after the addition of the acid to 1 liter water: pH = -Log 0.005 = 2.3 (a variation of 7 - 2.3 = 4.7 pH units). The buffering ability of the above solution depends on the fact that the strong acidity introduced with HCl is transformed into the weak acidity of CH3COOH. The addition of a strong base (e.g., NaOH) will produce the formation of the weak base CH3COO- with a little change of pH. The maximum buffering capacity of a given acid-base pair is then at the pH corresponding to the pK of the weak component, i.e., at pH where [AH] = [A-]. Buffering is still good in the pH range pKa ± 1. Finally, let's calculate the variation in pH after a ten fold dilution of the solution described above. From the Henderson-Hasselbach results that pH depends on the ratio Acetate/Acetic Acid, because the dilution affects these concentrations in the same way (Ca = Ca/10, Cb = Cb/10) there will be any variation in pH.
|
[CH3COOH] = [base] = 0.05 [CH3COO-] = [AH] initial - [base]
pH = 4.75 - Log (0.045) + Log (0.05) = 4.79
2. CH3COO- + H+ --> CH3COOH [CH3COOH] = 0.045 + 0.005 = 0.05 [CH3COO-] = 0.05 - 0.005 = 0.045 pH = 4.75 - Log (0.05) + Log (0.045) = 4.70. |
|
Example 1. Preparation of 1 liter of a sodium acetate buffer 0.1 M, pH 5. First we note that the concentration of a buffer refers, generally, to the analytical concentration of the weak component. In this case 0.1 M is the sum of [CH3COOH] and [CH3COO-]. The most simple procedure to prepare this buffer is to
This is the most simple procedure if we can measure the pH (e.g., with a pH-meter) of the solution during the addition of the base. If a pH-meter is not available, we can use the Henderson-Hasselbach equation to calculate the amount of NaOH (indicated with x) needed to reach pH 5 (see 1-7). Example 2. Let's see as the same buffer can be prepared using sodium acetate and acetic acid. According to the calculations made above we need to add 0.064 moles of NaOH to the acetic acid solution and thus [CH3COOH] = 1 - 0.064 = 0.036. Therefore,
If we dissolves 0,064 moles of sodium acetate and 0.036 moles of acetic
acid in 1 liter of water, we have that
For this reason you can find the equation of Henderson-Hasselbach written as in 8. |
6. 0.1 = 0.56x + x = x(0.56 + 1) 7. x = 0.1/1.56 = 0.064 moles of NaOH
Ca is the concentration of the acid. Cs is the concentration of the salt. |
 Earlier
on these page we have described the titration of Cl- with AgNO3
using chromate as indicator of endpoint of the titration. In the case of acid-base
reactions the monitoring of pH can be used to establish the endopoint of the
titration. The most simple acid-base titration is that involving a strong acid
and a strong base (e.g., HCl and NaOH). Since neither Na+ or Cl-
can hydrolyze, the endpoint of the titration is indicated by pH = 7. At this
pH, the number of moles of the base is equal to those of the acid (equivalence
point) and the concentration of the unknown can be calculated from the volume
of titrant added. In the case of a titration involving a weak acid with
a strong base or a strong acid with a weak base the hydrolysis phenomen should
be taken in account and the equivalence point, will be at pH >7 when
a weak acid is used and at pH <7 when a weak base is used. The simplest way
of estimating the pH of a solution is provided by the so called indicators.
These are weak acids or bases whose respective conjugate forms have a different
color. The color of a solution containing an indicator depends on the ratio
of conjugate forms, i.e., on pH value:
Earlier
on these page we have described the titration of Cl- with AgNO3
using chromate as indicator of endpoint of the titration. In the case of acid-base
reactions the monitoring of pH can be used to establish the endopoint of the
titration. The most simple acid-base titration is that involving a strong acid
and a strong base (e.g., HCl and NaOH). Since neither Na+ or Cl-
can hydrolyze, the endpoint of the titration is indicated by pH = 7. At this
pH, the number of moles of the base is equal to those of the acid (equivalence
point) and the concentration of the unknown can be calculated from the volume
of titrant added. In the case of a titration involving a weak acid with
a strong base or a strong acid with a weak base the hydrolysis phenomen should
be taken in account and the equivalence point, will be at pH >7 when
a weak acid is used and at pH <7 when a weak base is used. The simplest way
of estimating the pH of a solution is provided by the so called indicators.
These are weak acids or bases whose respective conjugate forms have a different
color. The color of a solution containing an indicator depends on the ratio
of conjugate forms, i.e., on pH value:
|
|
[AH] pH = pKa - Log ------- [A-] |
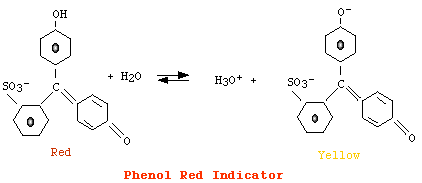
For phenol red
[AH] > [A-], color = red |
However, since eye can detect changes in color only when the concentration of a form is ten fold the concentration of its conjugate, we have that:
the color corresponding to [AH] is detected when pH = pKa + Log (0.1) i.e., when pH = pKa - 1the color corresponding to [A-] is detected when pH = pKa + Log (10) i.e., when pH = pKa + 1
|
In order to illustrate the use of indicators, here we calculate the variations of pH during the titration of HCl with NaOH. For the meaning of symbols see window on the right. After each addition the remaining moles of H30+ are given by
Va Ma Vb Mb By dividing with the total volume (Va + Vb = Vt) we get VaMa - VbMb The formula above allows to calculate the concentration of H30+ and then the pH after each addition of NaOH. The picture on the right reports variation of pH versus moles (or the equivalent volume) of NaOH during the titration of 100 ml of 0.1M HCl with 0.1 M NaOH. The most important feature of the titration curve is the rapid change of pH near the equivalence point (moles of acid = moles of base). It can be calculated that in going from 99 ml of base added to 100.5 ml there is a variation of about 7 pH units (from 3.3 to 10.4). Thus any indicator which changes color in this pH range (e.g., phenolphthalein or methyl Red) can be used to determine the endpoint of titration. Molecular weight determination. Acid-base titrations can be also used to determine the molecular weight of unknown weak acids and bases. A weighted amount of the unknown is titrated with an acid or a base (of known concentration) until the equivalence point is reached. At the equivalence point the moles of the titrant added are equal to moles of the unknown substance, thus the molecular weight can be determined by means of the relation Molecular weight = grams / molespK determination. The titration curve of a weak acid (base) with a strong base (acid) is well described by the Henderson-Hasselbach equation, for a weak acid pH = pK + Log [A-]/[AH]During the titration we can assume with a good approximation that [A-] is equal to the moles of the base added and [AH] is equal to the difference between the initial concentration of the acid and the moles of the base added. By plotting the pH versus the Log [A-]/[AH] we obtain a straight line which intercepts the pH axis at a value corresponding to pK. The determination of the molecular weight and pK values of an unknown compound can allow its identification. |
Ma = molarity acid
Mb = molarity base
Reload this image if animation stops. |