Gases
Starting from 1660, some simple laws describing the behavior of gases were
experimentally determined. Such laws can be mathematically deduced (kinetic
theory of gases) assuming that
1. The volume occupied by molecules is negligible when compared
to the volume occupied by the gas. Therefore the volume occupied by the gas
is the volume really available to gas molecules for their movements.
2. There is no interactions between molecules (except for
brief moments).
The laws which can be deduced, when the assumptions above hold (generally for
gases at low values of pressure), are known as laws of ideal gases.
|
Boyle's law states that at constant temperature (isothermal
process) the volume of a gas is inversely proportional
to its pressure. In other words, an increase of pressure
leads to a decrease of the volume of a gas and viceversa.
In mathematical terms the law of Boyle can be expressed by the following
equation
PV = const (at constant T)
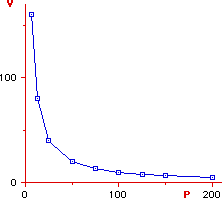
which is the equation of an hyperbola (see fig). If the
plotting of P vs V yields an hyperbola than we can affirm that the
gas is ideal otherwise the gas is nonideal (imperfect) at the
given temperature. The plot can be made at different temperatures thus
obtaining a family of curves called isotherms. If all curves are
hyperbolas the gas obeys Boyle's law at the given temperatures.
|
 |
|
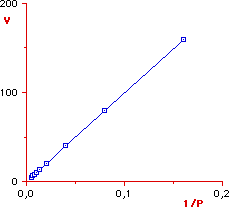
Since deviations from a straight line can be more easily
detected, it is very useful to transform the equation of
Boyle in such a way to obtain the equation of a straight line. The Boyle's
equation can be solved for V thus obtaining
 1 1
 V = const
--- V = const
---
 P P
By plotting V
versus 1/P (or P versus 1/V), we obtain a straight line with
slope = const. Therefore, a gas is ideal when the plot of V
versus 1/P (or P versus 1/V) yields a straight line.
|
 |
|
Suppose to have a gas at pressure P1 and volume
V1, if we change the pressure, at constant temperature, from
the value P1 to the value P2 we will have a change
in volume from V1 to V2 and the following equation
must hold
P1V1 = P2V2 = const
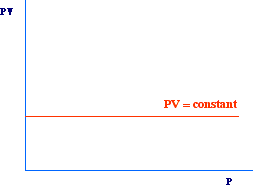
According to this equation, another simple way to obtain a straight line,
is to plot PV versus P (or versus V). In this case we will obtain
a straight line with slope zero, i.e., parallel to x axis. In fact, for
any value of P the PV product should have the same value.
|
 |
Laws
of Charles and Gay-Lussac
|
This law states that at constant pressure, the volume of a gas increases
linearly with the temperature. In mathematical terms, the laws of
Charles and Gay-Lussac is expressed by the following equation:
|
V = Vo(1 + at)
= Vo + Voat
where
V = volume of a fixed amount of gas
Vo = volume the gas occupies at 0 oC
t = temperature on the celsius scale
a = constant.
|
|
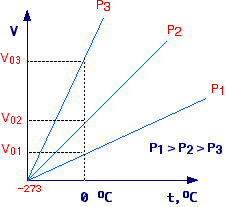
When plotted, this equation yields a straight line with slope
= Voa.
By plotting experimental values of V versus t, at different constant pressures,
a family of straight lines is obtained. For each line, it can be experimentally
determined that
slope = Vo / 273.15 hence a
= 1/273.15.
Furthermore, each line start from a value of -273.15 °C,
in fact for V = 0
Voat = - Vo
from which t = -1/a = -273.15.
Let's consider now a gas a two differents temperature:
|
t1
|
t2
|
By dividing V1 with V2
|
|
V1 = Vo(1 + at1)
|
V2 = Vo(1 + at2)
|
 V 1 (1
+ at 1)
 --- = -----------
 V 2 (1
+ at 2) |
by dividing numerator and denominator with a
(= 1/273.15) we obtain:
 V1
V1 (273.15
+ t1)
(273.15
+ t1)
 --- = ----------------
--- = ----------------
 V2
V2 (273.15
+ t2)
(273.15
+ t2)
The term 273.15 + t defines the absolute temperature (Kelvin scale,
K), i.e.,
T (absolute temperature) = 273.15 + t
By using T in place of 273.15 + t we get:
V1 T1 T1
--- = --- = const
V2 T2 T2 |
V
--- = const
T |
V = (T) (const) |
From the last equation we can deduce that the volume of a gas should
be zero at 0 K (-273.15 oC) and since the volume of a
gas can not be negative thus 0 K should be the lowest temperature possible.
By combining the Boyle's law and the law of Charles and Gay-Lussac, it can
be derived that
 T
T
 V = ----
const
V = ----
const
 P
P
This equation states that the volume of a gas increases linearly with the
temperature and decreases linearly with the pressure.
Considering that the volume of a gas depends also on its amount, and taking
the moles as unit of measure, we have that const = nR where R
is a new constant known as the universal gas constant. The equation
above can be then rearranged as follows
 nRT nRT
V = -----
 P P |
PV = nRT |
PV
----- = nR = const
T |
P1V1 P2V2 P2V2
------ = ------
T1 T2 T2 |
It is interesting to note that for
| T = const |
P1V1 = P2V2
, Boyle's law |
| P = const |
V1/T1 = V2/T2;
V1/V2 = T1/T2 , Gay-Lussac Law |
| V = const |
P1/T1 = P2/T2;
P1/P2 = T1/T2 , Charles Law |
The Avogadro law states that different gases at the same values
of P, V and T contain the same number of moles.
In fact, considering two gases (a and b) at the the same values of
P, V and T, we can write:
Since P,V,T are the same and R is a constant, then na = nb.
It follows that a given amount of different gases have the same volume
when subjected to the same pressure and temperature. The volume of
one mole of a gas at 0 oC (273.15 K) and 1 atm has been experimentally
measured and resulted to be 22.41 liters. This volume is known as the
standard molar volume and the condition of temperature and pressure
at which it has been determined are known as standard condition and generally
abbreviated as STP.
Considering the standard conditions (1 atm and 0°C) and 1 mole of
gas, we can easily calculate the value of R :
 PV
PV (1
atm) (22.414 liters)
(1
atm) (22.414 liters) (atm)(liters)
(atm)(liters)
R = ----- = ------------------------- = 0.08205 -----------------
 nT
nT  (1
mole) (273.15 K)
(1
mole) (273.15 K) (moles)
(K)
(moles)
(K)
Remembering that the pressure is given by force/area and area
and volume can be expressed in terms of length (L), we have
 PV
PV F
V
F
V F L3
F L3 FL
FL
R = ----- = ------ = ------ = ----
 nT
nT A
nT
A
nT L2 nT
L2 nT nT
nT
Since the product of a force with a length is the work which have the same
dimensions as energy, we have that the dimension of R, in the SI system, are
energy Joule
Joule
-------- = -----------
mole K  kg-mole
K
kg-mole
K
It should be evident that the numerical value of R depends on
the units used to measure P, V, T. In the appendix you can find a table
reporting the values of R when using different units.
Sometimes, when working with a particular gas, it is convenient to include
the molecular weight of the gas in R. Remembering that moles = mass/molecular
weight, we have
 mass
mass
PV = nRT = ------ RT
 mw
mw
4 and indicating with R' the ratio of R with mw we have
PV = mass R' T
where R' is specific for the gas considered.
Dalton's law states that in a mixture of gases each gas exerts the same
pressure as it would exerts if it was present alone. Let consider
two different gases (1 and 2)
|
Gas 1
in a vessel with volume V, exerting the pressure
p1
 RT RT
 p1 =
n1 --- p1 =
n1 ---
 V V
|
Gas 2
in a vessel with volume V, exerting the pressure
p2
 RT RT
 p2 =
n2 ---- p2 =
n2 ----
 V V
|
When the two gases are mixed together in a vessel of volume V, the pressure
exerted by gas 1 is still p1 and the pressure
exerted by gas 2 is still p2. It follows that
the total pressure, exerted by the two gas is Pt = p1
+ p2 and it is given:
 RT
RT RT
RT
 Pt = p1
+ p2 = (n1 + n2) ---- = nt ----
Pt = p1
+ p2 = (n1 + n2) ---- = nt ----
 V
V V
V
p1 and p2 are commonly known as partial pressures.
By dividing the partial pressure of one of the two gases (e.g., gas 1) with
the total pressure (equ. above) we get
 p1 p1 RT RT V V n1 n1
 ---- = n1
---- ------- = ----- ---- = n1
---- ------- = -----
 Pt Pt V V RT
nt RT
nt nt nt |
 n1 n1
 p1 = ------
Pt = xPt p1 = ------
Pt = xPt
 nt nt |
where x is the mole fraction (n1/nt) of gas 1. In words,
the partial pressure exerted by a gas in a mixture is given by the product of
its mole fraction with the total pressure (p = xPt).
According to Avogadro law, at the same condition of temperature and
pressure, the volume occupied by any gas depends only on its number of moles
thus
p1/Pt = n1/nt = V1/Vt
these ratios are known as pressure fraction, volume fraction and mole fraction
respectively.
The concept above can be applied to any number of ideal gases.
Consider a mixture of two gas of mass Mt, remembering
that moles = mass/molecular weight, we can write
Mt = n1 Mw1 + n2 Mw2
by dividing with nt (total moles), we have:
Mt n1
n2
--- = ---- Mw1 + ---- Mw2 = x1Mw1
+ x2Mw2
nt nt
nt
The term Mt/nt represents the mass of one mole
of the gas mixture, i.e., the average molecular weight of the mixture, therefore
Average Mw = x1Mw1 + x2Mw2
This formula can be applied to mixtures containing any number of gases:
Average Mw = x1Mw1 + x2Mw2 +
..... xnMwn
We have seen before that the term PV has the dimensions of a work (energy),
therefore when a gas expand (or is compressed) a work is done (see thermodynamics
for more). Let's consider the heating of a gas, in two differents conditions:
1. The gas is heated by maintaining a constant pressure, in this
condition the gas expands thus doing a work (PDV).
Therefore, at constant pressure, the energy supplied will be part transformed
in work and part transformed in kinetic energy
thus increasing the temperature of the gas.
2. The gas is heated by maintaining a constant volume, in this
condition no work can be accomplished (DV
= 0) and all the energy supplied will be transformed into kinetic energy.
The energy needed to raise 1 mole of the gas one degree, is referred
to as heat capacity. When this energy is referred to 1
gram (kilogram) of a substance the term specific heat is used. By
indicating with Cp and Cv the heat capacity
(specific heat) at constant pressure and constant volume respectively we
have that
Therefore, in constant pressure process, more energy is to be added to
observe the same change of temperature. It can be demonstrated (kinetic theory
of gas) that for a monoatomic gas, the variaton of kinetic energy with temperature
is given by DE
= 3/2 (R DT). From this equation, we have
that DE/DT =
3/2 (R), DE/DT
is the energy for unit temperature and thus represents the heat capacity. Therefore
At constant volume: Cv = DE/DT
= 3/2 (R) = 3/2 (1.987 cal/mol oC) = 2.97 cal/mol oC.
At constant pressure: Cp = Cv + PDV
= Cv + P (V2-V1) = R (T2-T1)
= R DT
Since we are dealing with heat capacity then DT
= 1 then Cp = Cv + R = 3/2 (R) + R = 5/2 (R)
From the data above we can calculate the theoretical valuer of Cp/Cv,
which is
Cp/Cv = [5/2 (R)] / [3/2 (R)] = 5/3 = 1.67
For monoatomic gas (e.g. He, Ne, Kr), the experimentally determined ratio is
very close to 1.67. For diatomic gas (e.g., H2, N2, O2)
the ratio Cp/Cv is significantly less than 1.67 (about 1.4).
| We have seen that for an ideal gas equ 1 and
2 hold. The quantity PV/RT which is 1 when 1 mole is considered,
is known as the compressibility factor (generally indicated as
z).
In fig 1, is reported a qualitative plot of z versus P,
it can be observed that
- For ideal gases a straight line parallel to the axis
of pressure is obtained, i.e., z is constant (see 2).
- For real gases, z is not constant and its value depends
on pressure values and on the nature of the gas. Furthermore, at low
pressure the value of z approaches the unit.
In order to understand such deviations, we should reconsider the assumptions
made for the deduction of laws of ideal gases.
|
|
1. PV = nRT
|
2. n = PV/RT = z
|
Fig 1.
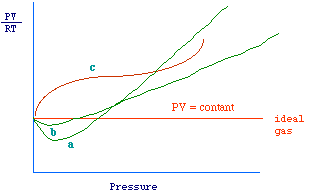
|
The first assumption was that the volume occupied by molecules of an ideal
gas is negligible when compared to the volume occupied by the gas. Therefore
the volume occupied by the gas is the volume really available to gas molecules
for their movements. For a real gas the volume occupied by molecules
can not be neglected and thus indicating with b the volume occupied by
molecules (known as covolume), the volume really available to molecules
should be V - b.
The second assumption was that there is no interactions between molecules.
For a real gas the attraction between molecules ("internal pressure"
) leads to a value of pressure which is lesser than that theoretically predictable.
Therefore, during the compression of a real gas, the deviation from unit
depends on the balance of two contrastant effects:
Because of covolume, the reduction of the volume should be lesser
than that predict by PV = nRT.
Because of internal pressure, the reduction of the volume should
be greater than that predict by PV = nRT.
The behavior of real gas can be, with a moderate accuracy, described by van
der Waals equation:
(P + a/V2) (V - b) = RT
where a and b are positive constants characteristic for a given
gas.
When the pressure is very low V tends to be much larger than b and a/V2
tends toward zero and thus the equation reduces to PV = RT.
|
Let's consider again the plot z vs. P, it should be evident that below
the ideal line (z < 1), the effect of internal pressure is more
important than the effect of covolume (greater compressibility).
Above the ideal line (z > 1) the effect of covolume is more important
than that of internal pressure (minor compressibility).
Furthermore, for lines a and b, there exist a value of pressure
at which the effect of covolume becomes more important than that of internal
pressure.
|

van der Waals: (P + a/V2)
(V - b) = RT
|
The inversion can be easily explained by noting that the higher the pressure
the lesser the volume and thus the effect of covolume (which is constant) becomes
more important. The pressure at which the inversion occurs is known as inversion
pressure. As for pressure there exists a temperature of inversion. Above
this temperature the effect of covolume is more important than that due to internal
pressure. Of course at the inversion pressure and temperature the gas behaves
as an ideal gas. Remembering that the product PV is a work, we can deduce that
an ideal gas (PV = constant) does not accomplish any work under compression
or expansion (thus T = constant).
A real gas, instead, when the pressure is changed can accomplish a positive
or a negative work depending on the relative importance of covolume and internal
pressure. Let's consider a gas at a temperature above the inversion temperature,
i.e. at a temperature for which the effect of covolume is more important than
that of internal pressure. From the figure is easy to verify that by increasing
the pressure from P1 to P2 it will be P2V2
- P1V1 > 0. Therefore under compression the gas accomplish
a work with a consequent decrease of its internal energy and than of its temperature.
Of course when the gas is allowed to expand its temperature increases.
For a gas below its inversion temperature (and its inversion pressure) the
compression will increase the temperature while an expansion decreases it.
Let's consider the isothermic compression of carbon dioxide at temperatures
less than 31.3 oC:
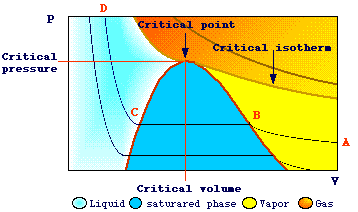 At
relatively low values of pressure, as the pressure increases, there is a significant
decrease of volume (line A-B) .
At
relatively low values of pressure, as the pressure increases, there is a significant
decrease of volume (line A-B) .
When the point B is reached, condensation begins and we can observe a decrease
in volume at constant pressure (line B-C). At point B the gas is in a so called
state of saturation, i.e. it is in equilibrium with its liquid phase.
At point C, where only the liquid state occurs, the increase in pressure results
in a small decrease in volume.
If the compression is performed at higher temperatures (less than 31.3oC),
the same behavior will be observed but the change in volume, during the phase
change, will be less than that observed before.
When the compression is performed above 31.3oC (critical isotherm)
no liquid will form and there is only a decrease in volume and a corresponding
increase in density.
The maximum value of temperature at which condensation can occur is said critical
temperature.
The corresponding values of pressure, volume, density are also said critical.
When a liquid is compressed above its critical pressure and then heated to
a temperature above its critical temperature a superheated fluid is obtained
without boiling. In this process two phases (liquid and gas) are never observed.
The fluid obtained in this way is said supercritical fluid.





 At
relatively low values of pressure, as the pressure increases, there is a significant
decrease of volume (line A-B) .
At
relatively low values of pressure, as the pressure increases, there is a significant
decrease of volume (line A-B) .