Phase changes
Matter can exist in three aggregate states, i.e., solid, liquid and gas. The
passages from one state to another (by adding or removing heat) are known as
phase changes. The transformation from the solid state to the liquid state is
said fusion or melting, the inverse is said solidification.
The transformation from the liquid state to the vapor state is said vaporizzation,
the inverse is said condensation. Some substances can pass from the solid state
directly to the vapor state (i.e., without passing from the liquid state) and
viceversa. The term sublimation is used to indicate such transormations.
Fusion is the passage from the solid state to the liquid
state and it requires heat (energy) to take place.
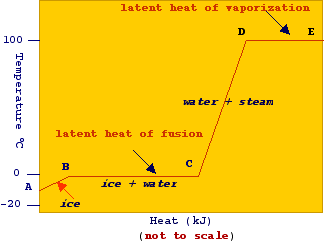 Let's
consider the heating of ice (1 atm) from -10 oC to 0 oC,
at beginning we can observe an increase of temperature and when the temperature
reach 0 oC, fusion (melting) begins (formation of liquid water).
The temperature at which fusion begins (0 oC and 1 atm, in the case
of water) is said fusion (melting) temperature. By further addition of
heat, until ice and water coexists, no change of temperature is observed (line
B-C in figure). Once all ice is transformed into water (point C) the temperature
starts to increase again.
Let's
consider the heating of ice (1 atm) from -10 oC to 0 oC,
at beginning we can observe an increase of temperature and when the temperature
reach 0 oC, fusion (melting) begins (formation of liquid water).
The temperature at which fusion begins (0 oC and 1 atm, in the case
of water) is said fusion (melting) temperature. By further addition of
heat, until ice and water coexists, no change of temperature is observed (line
B-C in figure). Once all ice is transformed into water (point C) the temperature
starts to increase again.
The heat which does not produce a temperature change is said
latent heat of fusion (it is used to breakdown attractive forces between
molecules and no temperature change is observed). On the contrary, the heat
which produces a temperature change is said sensible heat
(line A-B).
 |
Notes on fusion
1. Not all substances has a constant temperature of fusion,
in fact a constant temperature is observed only for crystalline solids
but not for amorphs (not crystalline) solids such as glass, wax, etc.
2. For some substances (e.g., wood, bones) no fusion can
be observed, they just decompose whe heat is supplied.
3. For most substances the volume of the liquid phase is
greater than that of the solid phase. Water is an important exception.
4. The fusion temperature depends on pressure.
When the volume of the liquid phase is greater than that of the solid
phase, an increase of pressure leads to an increase of the fusion
temperature.
Otherwise (e.g., water) an increase of pressure lead to a diminution
of the fusion temperature.
The effect of pressure on the fusion temperature can be explained on
thermodynamics base (see later, equation of Clausius-Clapeyron)
|
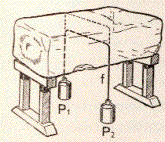 |
A simple experiment can demonstrate that
the fusion temperature of ice decreases when pressure increases. Consider
a block of ice on which is placed a very thin string of steel having at
the extremities two big weights (see figure). Since the surface of contact
between ice and steel is very small, weights will generate a considerable
increase of pressure at surface contact. Since an increase of pressure leads
to a decrease of the fusion temperature ice becomes liquid and the string
moves down. Liquid water is now above the string and it experiences the
normal pressure and solidification occurs. Therefore the string will pass
through the ice block without dividing it in two parts. |
 The process of solidification can be illustrated with the same graph used for
fusion. Consider the cooling of liquid water (any point on the line CD), we
will observe a decrease of temperature until we reach point C at which water
begin to solidify (the corresponding temperature is the temperature of solidification
which matches the temperature of fusion). By further cooling, the temperature
will remain constant untill all liquid water is transformed in ice. The heat
needed to transform all water in ice is said latent heat of solidification which
is equal to the latent heat of fusion.
The process of solidification can be illustrated with the same graph used for
fusion. Consider the cooling of liquid water (any point on the line CD), we
will observe a decrease of temperature until we reach point C at which water
begin to solidify (the corresponding temperature is the temperature of solidification
which matches the temperature of fusion). By further cooling, the temperature
will remain constant untill all liquid water is transformed in ice. The heat
needed to transform all water in ice is said latent heat of solidification which
is equal to the latent heat of fusion.
For some substances, when cooled slowely, it is possible to exceed the solidification
temperature (at a given pressure) without observing the formation of the solid.
Such liquids are said subcooled and are very unstable, in fact by means
of a light shaking it solidify reaching the solidification temperature (it warm
up).
Vaporization is the passage from the liquid state to the gas
(vapor) state and it requires heat (energy) to take place.
 Vaporization
in a close container.
Vaporization
in a close container.
When the evaporation takes place in a closed evacuated container at constant
temperature, we can observe that
only part of the liquid evaporates;
the pressure exerted by vapor molecules increases with time until to reach
a constant value.
These observations can be explained by considering that vapor molecules can
return to the liquid state (condensation) and the rate at which vapor
molecules condense depends on pressure (the higher the pressure the higher
the rate of condensation). At the beginning the rate of evaporation is greater
than the rate of condensation and we observe a net formation of vapor but as
the pressure increases the rate of condensation increases until to matches the
rate of evaporation and no more vapor is formed. When the liquid-vapor equilibrium
is reached we have that evaporation rate = condensation rate and the
pressure exerted by vapor is constant. This equilibrium pressure is known
as the vapor pressure. The concept of vapor pressure will
be deepen later on these pages. Here we just note that vapor pressure
measures the tendency of a liquid to enter the vapor state (the higher the
vapor pressure the higher the rate of evaporation) and it depends only
on temperature (the higher the temperature the higher the vapor pressure).
As an example of this last point we can compare the vapor pressure of three
commonly encountered liquids (values in torr at 30°C):
|
Water (the less volatile)
|
Ethyl alcohol
|
Acetone (the most volatile)
|
|
31.82
|
78.8
|
282.7
|
 Vaporization
in a open container.
Vaporization
in a open container.
A common experience is that liquids of relative low boiling temperature, placed
in a container open to the atmosphere, will ultimately evaporate entirely. This
can be explained by considering that
in order to enter vapor state, molecules need energy to break-down the
attractive forces of their neighbors molecules. In fact, when a volatile
liquid, e.g. ether, is applied on the skin we can determine the cooling of
the skin. This due to the fact that the energy needed for evaporation is taken
from the skin thus producing a diminution of temperature. Some liquids, such
as ethyl chloride can be used as local anesthetics for they freeze the skin
upon evaporation.
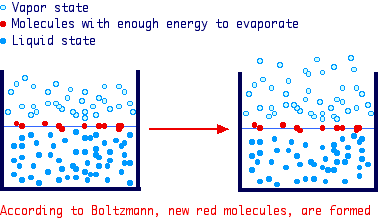
the energy of molecules in a liquid is distributed according to Maxwell-Boltzmann
law which can be briefly summarized as follows:
If E is the energy molecules should have to evaporate, the number of
molecules having an energy greater than E is proportional to e-E/kT.
At a given temperature, some molecule exist having enough energy to enter the
vapore state (red molecules). Once these molecules are lost in the atmosphere,
in the remaining liquid there will be a redistribution of the energy (according
to Boltzmann factor) and new molecules will have an energy greater than E and
evaporation continues until no liquid is left.
Normally, for a liquid in a container open to the atmosphere the pressure above
the liquid is due to the vapor molecules and to air. We can imagine
that, on the surface of the liquid there exists an envelope containing vapor
molecules and air. Suppose that the atmospheric pressure is 1 atm
(101.3 kPa). In 'absence' of vaporization the pressure exerted by air is
101.3 kPa and it is due only to air molecules. But, when vaporization
occurs part of air molecules are pushed out, by water molecules, from the
envelope above the liquid. The total pressure will be always 101.3
kPa but now it is due both to vapor and air molecules.
According to Dalton' law, we have that
Total pressure = Pair + Pvapor = 101.3 kPa
In the following table is reported the vapor pressure of water at different
temperatures along with the pressures of air calculated according to Dalton's
law:
|
T (oC)
|
P (kPa)
|
|
25
|
3.2
|
|
35
|
5.7
|
|
45
|
9.6
|
|
100
|
101.3
|
|
|
Air pressure
|
|
101.3 - 3.2 = 98.1
|
|
101.3 - 5.7 = 95.6
|
|
101.3 - 9.6 = 91.7
|
|
101.3 - 101.3 = 0
|
|
As can be seen, at 100oC, the vapor pressure of water is exactly
101.3 kPa and thus only water molecules will be in the space over the liquid.
At this temperature, known as boiling point, the liquid starts to boil
and a further administration of heat will not increase the temperature until
all water is evaporated.
The boiling process is characterized by the formation and the growt of bubbles
of vapor throughout the bulk of a liquid. The initiation of a bubble in a liquid
is a very difficult process, in fact it requires that many molecules of energy
greater than that needed for vaporization must be close one other. Thus, it
is not always guaranteed that a liquid starts to boil at its boiling temperature.
In such condition, the administration of further heat will produce a superheated
liquid, i.e. a liquid having a temperature greater than its boiling
point. When the formation of bubbles in a superheated liquid eventually occur,
it can be very violent and dangerous. In fact, since the vapor pressure in bubbles
greatly exceeds atmospheric pressure, the bubbles tend to expand rapidly. The
violent boiling (said bumping) can be avoided by introducing materials
which initiate bubbles in the liquid as soon as the boiling point is reached.
For example, porous pieces of ceramic which evolve small bubbles of air into
which evaporation can occur.
|
Notes: The pressure and temperature at which a phase change
takes place are said saturation pressure and saturation temperature
respectively. When a substance occurs in the liquid state at its
saturation conditions, it is said saturated liquid. As an example,
liquid water at 100 oC and 1 atm water starts to boil and it
is a saturated liquid. On the other hand the term saturated vapor
is used to indicate a substance that occurs in vapor state at its saturation
temperature and saturation pressure. Steam at 100 oC and 1
atm is a saturated vapor. If, after the complete transformation into vapor,
further heat is administered we obtain a vapor with a temperature greater
than its saturation temperature, i.e. a superheated vapor.
|
 Let's
consider the heating of ice (1 atm) from -10 oC to 0 oC,
at beginning we can observe an increase of temperature and when the temperature
reach 0 oC, fusion (melting) begins (formation of liquid water).
The temperature at which fusion begins (0 oC and 1 atm, in the case
of water) is said fusion (melting) temperature. By further addition of
heat, until ice and water coexists, no change of temperature is observed (line
B-C in figure). Once all ice is transformed into water (point C) the temperature
starts to increase again.
Let's
consider the heating of ice (1 atm) from -10 oC to 0 oC,
at beginning we can observe an increase of temperature and when the temperature
reach 0 oC, fusion (melting) begins (formation of liquid water).
The temperature at which fusion begins (0 oC and 1 atm, in the case
of water) is said fusion (melting) temperature. By further addition of
heat, until ice and water coexists, no change of temperature is observed (line
B-C in figure). Once all ice is transformed into water (point C) the temperature
starts to increase again.
