Thermodynamics
A system can be defined as the region of space or amount of matter on which
we make controlled experiments. The entities external to the system, which can
influence the state of the system, are known as surroundings. Systems
are distinguished in
close systems when they can exchange only energy with
surrounding;
Open systems when they can exchange both matter and energy
with surrounding;
Isolated systems when they can not exchange neither mass and
energy.
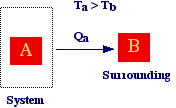 Consider
two bodies at differents temperature, if the body A (the system) can exchange
only heat with body B (the surrounding) then A is a close system. It
should be evident that the distinction between system and surrounding is arbitrary
and depends on our approach in studying a phenomenon. We could also consider,
in other cases, A the surrounding and B the system. Eventually, if body A interact
only with body B and viceversa the system formed by body A and B is an isolated
system.
Consider
two bodies at differents temperature, if the body A (the system) can exchange
only heat with body B (the surrounding) then A is a close system. It
should be evident that the distinction between system and surrounding is arbitrary
and depends on our approach in studying a phenomenon. We could also consider,
in other cases, A the surrounding and B the system. Eventually, if body A interact
only with body B and viceversa the system formed by body A and B is an isolated
system.
 Intensive
and extensive properties.
Intensive
and extensive properties.
The properties of a system can be distinguished into intensive and extensive
properties.
Intensive are those properties which are independent from the amount
of matter. Examples of intensive properties are temperature and density.
If we withdrawn some water from a bottle the temperature and the density
of water does not change.
Extensive are those properties which are dependent on the amount of
matter. Examples of extensive properties are volume and mass. If we withdrawn
some water from a bottle the volume of water in the bottle will reduce (as the
mass).
Intensive properties are of greater interest to chemists because they are much
more characteristic of the nature of a substance than extensive properties.
The division of an extensive property with another extensive property leads
to an intensive property. For example, density (m/V) is an intensive
properties (does not depend on the amount of substance) while both mass and
volume are extensive properties.
The volume of a given amount of any material (gas, liquid, solid) depends on
temperature and pressure value. An equation of state is the mathematical relation
describing the dependence of volume from pressure, temperature and the amount
(expressed in moles) of the substance considered. This relation is symbolically
expressed as
| V = V(P, T, n) |
where
P = absolute pressure
T = absolute temperature
n = number of moles
|
In words this equation states that the volume of a substance is some function
of pressure, temperature and the number of moles of a material. In the case
of solids and liquids the equation of state may be very complicated and may
differ considerably from one substance to another. On the contrary, the equation
of state of all gases is nearly the same. The most simple equation of state
for gases is the well known ideal gas equation:
 nRT
nRT
V = ----- or PV = nRT (see gases for more).
 P
P
A function of state is a property of a system which depends only on the state
of the system and not on the way the state is reached. Typical functions of
state are pressure, volume and temperature.
The most important properties of a function of state are
1. Assigning values to few functions of state of a system, all the other
functions can be determined. As an example, from equation of state of ideal
gases (PV = nRT) it should be evident that once assigned a value to T
and V the value of the pressure is univocally determined. Thus a function of
state depends only on the values of other functions of state.
2. The change of a function of state depends only from the initial and
final state of the system and not on the way the change was accomplished.
3. Furthermore, (see mathematics section), for a function of state it
is possible to write an exact differential.
Please note that, in the text, an infinitesimal variation of a function of
state is indicated with d (the differential operator) while the symbol d
is reserved to other functions.
Energy
is function of state
|
 Energy
of a system.
Energy
of a system.
As already stated, energy is a property of a system which can not be visualized
but experiences indicate that energy transfer corresponds to heat transfer and
work transfer. In other words, the energy of a system can be changed by heat
and work. The energy of a system can be distinguished in
External energy which is the energy of a system associated
with its position (potential, EP) and velocity (kinetic,
EK)
Internal energy (U) which is the energy associated to with atoms and
molecules forming the system.
Therefore, the energy of system is given by
E = U + EP + EK
Generally, we are interested to the variation of the energy of a system rather
than the absolute energy of a system. The total change of the energy of a system
in going from one state to another can be written as
DE = DU +
DEP+ DEK.
Let's imagine an experiment in which some energy is added to a system in such
a way it passes from the state A (lower energy) to the
state B (higher energy) and let's indicate the variation
of energy of the system with EB - EA. Now, consider
another experiment in which the system returns to the state A by delivering
energy, the variation of energy of the system is indicated now with EA
- EB. There are two possibilities regarding the absolute
variations of energy in the two processes, i.e.,
1. ABS(EB - EA) <> ABS(EA -
EB)
2. ABS(EB - EA ) = ABS(EA - EB)
If equ 1 holds, in going from A to B and then back from B to A we could
destroy or create energy. The creation of energy is very attractive, but all
the numerous attempts to achieve it have failed and it is. generally accepted
that energy can not be created or destroyed. Therefore, equ 2 expresses the
right assumption.
In words, when we go back to state A we should recover the same amount of energy
spent to go from A to B and thus the energy is conserved. Furthermore since
the variation of energy depends only on the initial and final state of the system,
energy is a function of state.
Work
is not a function of state
|
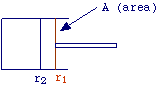
The most simple form of work that a system can exchange with surroundings is
that associated with variations of pressure and volume. Let's consider a gas
which expands against an external constant force (see fig.). When the piston
moves from the position r2 to position r1, work is given
by
| W = (fex) (r1 - r2) |
|
Multiplying and dividing with area, we have:
 fex (r1 -
r2) (A)
fex (r1 -
r2) (A)
 W = ----------------
W = ----------------
 (A)
(A)
remembering that f/A is a pressure and that the product of area for distance
is a volume, equation above reduces to
W = Pex (V1 - V2) = PexDV
It should be evident that when a gas expands it makes work at the expense
of its internal energy, i.e., its internal energy decreases. Conversely,
when we compress a gas, the work we make increases the internal energy of the
gas.
Consider now two different states for a gas (P1V1
and P2V2) with V2 > V1
and of course P1 > P2 (see
equation of state of an ideal gas). We can change the state of the gas (from
P1V1 to P2V2) in two
different ways:
1. The gas is first allowed to expand from V1 to V2
against the constant pressure P1 thus doing a work given by
W = P1 (V2 - V1)
succesively the pressure is lowered to P2 (keeping V2
constant). Of course, in this second part of the process, since no displacement
occurs W = 0.
2. The pressure on the gas is first lowered to P2 keeping
V1 constant and thus no work is accomplished. Successively,
the change in volume (V1 to V2) against the pressure P2
is allowed. In this case the work is
W = P2 (V2 - V1)
Since P1 and P2 are differents, a different amount of
work is made in the different paths described. Therefore, work is not a function
of state.
Heat
is not a function of state
|
Heat is defined as the energy transferred when a difference of temperature
exists between system and sorrounding. When a substance is heated, two phenomenona
can be generally observed:
1. an increase of temperature (an increase of internal energy)
2. an increase of volume (production of work, W = PDV).
As an example, let's consider the heating of a gas in two different conditions:
Constant pressure process. In this case part of the supplied
energy is transformed in work and part produces an increase of temperature (increase
of internal energy).
Constant volume process. When no change in volume is allowed,
no work can be accomplished and all the energy supplied is used to increase
the temperature.
It follows, that the same amount of heat produces a different variation of
the temperature (a state function) and thus heat is not a state function. However,
if we refer to a constant pressure process heat becomes a state function and
is said enthalpy (see later for more). Since most processes take place
at constant pressure, the terms enthalpy (H) and heat content are sometimes
used synonymously. If a system, under constant pressure, absorbs heat there
is an increase of its enthalpy, i.e., a positive change of its enthalpy (DH
> 0). Conversely, if the system evolves heat the change of enthalpy is negative
(DH < 0).
In the following table are reported the variation of enthalpy (the heat exchanged
under constant pressure) for the different phase changes of 1 mole of water
at 25°C and 1 atm:
|
Phase change
|
DH
|
Generally indicated as
|
Evaporation
(liquid to vapor)
|
10519 cal
|
H2O (l) --> H2O (g) DH
= 10519 cal
|
Condensation
(vapor to liquid)
|
-10519 cal
|
H2O (g) --> H2O (l) DH
= -10519 cal
|
Sublimation
(solid to vapor)
|
11955 cal
|
H2O (s) --> H2O (g) DH
= 11955 cal
|
Fusion
(solid to liquid)
|
1436 cal
|
H2O (s) --> H2O (l) DH
= 1436 cal
|
As can be observed, a solid can be transformed into its vapor by following
two different paths:
1. sublimation;
2. fusion of the solid first and successive evaporation of the liquid.
The energy required for sublimation is the sum of the energies required for
fusion and evaporation (11955 cal). This proves that enthalpy is a state function.
It can be easily experienced that the same amount of heat produces a different
increase of temperature in different substances. The amount of heat required
to raise the temperature of a fixed amount of a substance by 1 degree celsius
is referred to as specific heat. In the SI system specific heat is the amount
of energy (Joule, J) needed to raise 1 Kg of material one degree Kelvin. The
term heat capacity generally refers to 1 mole of substance.
The amount of heat needed to increase the temperature of a body of mass m from
temperature T1 to T2 is calculated by
Q = m C (T2 - T1)
Since the increase in temperature, of a given substance, depends on the way
the heating is accomplished (see above), we need to specify if the specific
heat was measured at constant pressure or at constant volume. These two quantities
are generally indicated with Cp (constant pressure) and Cv
(constant volume).
It is worth noting that specific heat depends on pressure and temperature.
However, for the moment, we consider constant pressure processes for which Cp
is constant in the range of temperature considered.
Furthermore, let's note that in the formula above we can use indifferently
temperatures in kelvin or in celsius. In fact the difference of 1 K corresponds
to a difference of 1°C.
 Consider
two bodies at differents temperature, if the body A (the system) can exchange
only heat with body B (the surrounding) then A is a close system. It
should be evident that the distinction between system and surrounding is arbitrary
and depends on our approach in studying a phenomenon. We could also consider,
in other cases, A the surrounding and B the system. Eventually, if body A interact
only with body B and viceversa the system formed by body A and B is an isolated
system.
Consider
two bodies at differents temperature, if the body A (the system) can exchange
only heat with body B (the surrounding) then A is a close system. It
should be evident that the distinction between system and surrounding is arbitrary
and depends on our approach in studying a phenomenon. We could also consider,
in other cases, A the surrounding and B the system. Eventually, if body A interact
only with body B and viceversa the system formed by body A and B is an isolated
system. 