|
Introduction
|
Carbohydrates is a generic term indicating organic compounds containing hydroxyl, ketonic and aldheydic groups. These compounds are produced by photosynthetic plants and contain only carbon, hydrogen, and oxygen, usually in the ratio 1/2/1. However the general formula Cn(H2O)n (hence "hydrates of carbon") is not always applicable.
The generic term carbohydrate includes monosaccharides, oligosaccharides and polysaccharides as well as substances derived from monosaccharides by reduction/oxidation (alditols, aldaric acids, etc.) reactions or by replacement of one or more hydroxyl group(s) by a hydrogen atom, an amino group, thiol group or similar groups.
Carbohydrates are generally distinguished in simple carbohydrates (monosaccharides and disaccharides) and complex carbohydrates (polysaccharides).
Monosaccharides is a general term indicating polyhydroxyaldehydes (aldoses) and polyhydroxyketones (ketoses) with at least two hydroxyl groups. They are generally indicated, depending on the number of carbon atoms, as thrioses, tetroses, pentoses, hexoses, etc.
The most simple carbohydrates are glyceraldehyde and dihydroxyacetone. Glyceraldehyde has an asymmetric carbon atom and thus two steroisomers (D-Glyceraldehyde and L-Glyceraldehyde) exist.
Two common tetrose are D-erytrose and D-threose, the prefix D means that the configuration of the asymmetric carbon atom farthermost from the aldehydic or ketonic group is the same as D-glyceraldehyde.
Two common hexoses (6 carbon atoms) are D-glucose and D-fructose. Also in this case the prefix D refers to the configuration of the asymmetric carbon atom farthermost from the aldehydic or ketonic group. In solution, the predominant structure of glucose is a cycle arising from the reaction of the hydroxyl group in position 5 with the aldehydic group (an intramolecular hemiacetals). The cycle for its resemblance with pyran is named glucopyranose.
In the case of fructose the the hydroxyl group in position 5 reacts with the ketonic group to give an intramolekular hemiketals. The cycle for its resemblance with furan is named fructofuranose.
The cyclic structures given above for glucose and fructose are known as projections of Haworth. Taking as an example glucopyranose we illustrate the main feature of the formula.
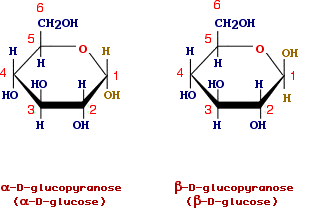
The cycle is planar and should be imagined to be perpendicular to the screen with the bolded line being the closest to the observeur. After the formation of the cycle the carbon in position 1 becomes asymmetric and the OH group can be below the plane of the ring (alpha-form) or above the plane of the ring (beta-form). The carbon in position 1 is named anomeric and alpha and beta forms are said anomers. In the case of fructose the forms alpha and beta refers to the position of the hydroxyl group bound to anomeric carbon at position 2.
In solutions, the open and cyclic structures readily interconvert and the term glucose (fructose) is used to refers to the mixture of the differents forms. When crystalls of alpha-D-glucose are dissolved in water we can observe that initially the specific optical rotation is 112° and decreases with the time until to reach a value of 52.5°. This phenomenon, known as mutarotation, is due to the interconversion of the differents forms until an equilibrium is reached which corresponds to 64% of beta anomer and 36 of the alpha. Conversely the solution of the beta anomer has an initial specific optical rotation of 19°. When the equilibrium is reached we can measure the same final value obtained forthe alpha anomer (52.5°).
As said above, the open and cyclic structures readily interconvert and a significant amount of sugar exists in the carbonyl form and thus they undergo to typical reactions of the carbonyl group. For example, they are able to reduce the reactive of Fehling or the Tollens' reagent and are generally referred to as reducing sugars.
The reactive of Fehling is an alkaline, blue solution of Cu2+ complexed with tartrate. In the presence of reducing sugars Cu2+ is reduced to Cu+ which precipitates as Cu2O (red) and the blue colour of the Fehling solution (due to the complex tartrate-Cu2+) disappears.
The Tollens' reagent: silver ion in ammonia solution.
Most of monosaccharides and disaccharides are reducing sugars. However when the anomeric carbon is involved in a chemical bond no free carbonyl group can exist and no reduction of the reagents above can be observed and the sugars are referred to as non-reducing sugars.
![]() Reduction
reactions
Reduction
reactions
Reduction of sugars with a mild reducing agents (e.g. sodium borohydride) allows to reduce the carbonyl group to alcohol, the resulting compounds (polyhydric alcohols) having the general formula HOCH2[CH(OH)] nCH2OH are known as alditols (glycitols is an obsolescent synonym for alditols) and named pentitols, hexitols, etc. Some of these are natural compounds, e.g., mannitol, glucitol (or sorbitol) and ribitol. Inositols (myo-inositol and scyllo-inositol) are cyclic hexitols.
The oxidation of aldoses can lead to the formation of three different kinds of carboxylic acids.
1) Aldonic acids in which the aldehydic group in C1 is oxidized to COOH. This oxidation can be accomplished by bromine. An example is gluconic acid (derived from glucose)
2. Aldaric acids in which also the primary alcohol function is is oxidized to COOH. This can be accomplished by a stronger oxidizing agent such as nitric acid. The general formula of aldaric acids is then HOOC - (CHOH)n - COOH, i.e. they are polyhydroxy dicarboxylic acids. An example is glucaric acid (derived from glucose).
3. Uronic acids are derived by the specific oxidation to a carboxy group of the terminal -CH2OH (the primary alcohol) group. An example is glucaronic acid (derived from glucose).
![]() Glycosidic
bond
Glycosidic
bond
An important reaction of carbohydrates is that with alcohol in presence of a inorganic acid which leads to glycosides formation. As an example, when glucose is allowed to react with methanol in presence of HCl, the anomeric hydroxyl group is replaced by a methoxyl group with formathion of methyl alpha and methyl beta-D-glucopyranosides. The glycoside is an acetal, in fact it contains two ethereal functions bound to same carbon atom (C-1) i.e., OCH3 and O-CH-CH2OH. The non-sugar moiety of glycosides, i.e., the group replacing the hydrogen of the OH at C-1 is named aglycone (in our example the methyl group is the aglycone). Furthermore, the bond between the sugar and the aglycone is named glycosidic bond. This bond is also responsible of the formation of disaccharides (see later).
Saccharides is a general therm indicating monosaccharides di-, oligo-, and polysaccharides. The term saccharides is considered by some to be synonymous with carbohydrates. In di, oligo and polysaccharides, monosaccharides are linked by glycosidic bonds. When only one of the two anomeric carbons is involved in the linkage the resulting disaccharide is denoted as glycosylglycose. Glycosyl refers to the moyety using its anomeric carbon in the glycosidic linkage while glycose refers to the reducing moiety (having a free anomeric carbon). Instead, when both the anomeric carbons are involved in the glycosidic bond, the disaccharide is denoted as glycosylglycoside.
In order to name a disaccharides we must also consider
the nature of constituent monosaccharides and the ring type as they exist (furanose or pyranose);
the carbon atoms involved in the linkage and the anomeric nature (alpha or beta) of the linkage.
![]() Lactose
Lactose
is the main disaccharide of milk, is formed through the formation of a glycosidic bond between the anomeric carbon of galactose and the OH in C-4 of glucose and thus lactose is a galactoside and should be named galactosylglucose.
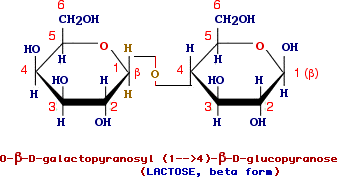
According to what said above the complete name for lactose is O-alpha-D galactopyranosyl (1-->4)-beta-D-glucopyranose. Since the anomer carbon of glucose is free, lactose can exist in alpha and beta forms and of course it is a reducing sugar and shows mutarotation upon dissolution.
![]() (+) Sucrose
(+) Sucrose
is the most abundant disaccharide (the common table sugar) is made up of alpha-D-glucopyranose and beta-D-fructofuranose which are bound through a glycosidic bond between the C-1 (alpha) of glucose and the C-2 (beta) of fructose.
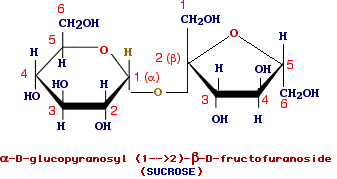
Therefore sucrose is a glycosylglycoside and its complete name should be alpha-D-glucopyranosyl (1-->2) beta-D-fructofuranoside but since fructose also uses its anomeric carbon for the glycosidic linkage the name beta-D-fructofuranosyl (2-->1) alpha-D-glucopyranoside can be indifferently used.
It is worth to note that in sucrose both the anomeric carbon are involved in the formation of the glycosidic bond and thus, differing from most disaccharides, sucrose is a non-reducing sugar and does not shows mutarotation when dissolved.
When (+) sucrose (specific optical rotation = 66.5°) is hydrolized by diluted acid or by the enzyme invertase (b-D-Fructofuranosidase) , the optical rotation becomes negative. This phenomenon is known as inversion and the resulting solution, formed by D(+) glucose and D(-) fructose, is known as invert sugar. The inversion is due to the fact that the specific optical rotation of D(+) glucose is 52.7° while that of D(-) fructose is -92.4°. For this reason, D(+) glucose and D(-) fructose are also known as dextrose and levulose respectively.
![]() Maltose
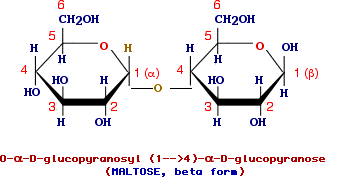
Maltose
is a disaccharide made up of two glucose and only one of the two available anomeric carbons is involved in the glycosidic linkage and thus maltose is a glucopyranosylglucopyranose and the complete name is O-alpha-D-glucopyranosyl (1-4)-alpha-D.
Glycogen and starch are the most common complex carbohydrates (polysaccharides), they are are branched polymers of glucose in which the glucose units are joined together by alpha 1-4 linkages.
They differs for the extent of branching (by alpha 1-6 linkages). Starch is composed by amylose and amylopectin which differs for the extent of branching (amylose has no branching).
Glycogen and starch are energy reserves in animals and plant respectively. In animals, glycogen is accumulated in liver and muscles.