|
Introduction
|
The first theory for chemical bond was based on the assumption that an atom is formed by a positive charged nucleus and by electrons moving in concentric orbits around a nucleus. Each orbit corresponds to an energetic level and can contain only a defined number of electrons (two in the first, eight in the second, etc.). At that time it was realised that an atom reaches the maximum stability when its external orbit is complete, i.e., it contains the maximum number of electrons allowed (as in noble gases).
![]() Ionic bond
Ionic bond
In ionic bonds there is a transfer of electrons between two atoms as in the case of lithium fluoride (LiF).
Consider the electron configuration of lithium and fluorine:
| Lithium | Fluorine |
| 1s2 2s1 | 1s2 2s22p5 |
Lithium has one electron in the external strate (2s1) and tends to lose this electron to have an external strate complete (1s2) as in the noble gas helium. In other words, lithium can reach a more stable state by losing an electron thus becoming positively charged:
Li ===> Li+ + e-
On the other hand fluorine has seven electrons in the external strate (2s22p5) which is complete when it contains eight electrons. Therefore, fluorine has the tendency to gain an electron, becoming negatively charged:
F + e- ===> F-
The electrostatic attraction between the two ions represents the ionic bond responsible for the formation of lithium fluoride.
![]() Covalent bond
Covalent bond
In covalent bonds atoms share electrons.
Let's consider the hydrogen molecule (H2). The electron configuration of hydrogen atom is 1s1 and thus the atom tends to gain an electron to reach the electron configuration of helium (1s2). In the hydrogen molecule the two atoms share their electrons thus reaching the configuration of helium.
According to quantum mechanic, it is not possible to know with precision the position and the velocity of an electron at a given instant. We can only know the probability to find an electron in a given space. The region of space in which is probable to find an electron in an atom is known as atomic orbital (molecular orbital when we deal with molecules). The orbital can be imagined as a not uniform cloud which is more dense in regions where the probability to find the electron is higher. An orbital does not have a well defined boundary for there is a finite probability to find an electron very far from the nucleus. However, the greater the distance the lower the probability and the form of orbitals given below are a good approximation of the true form.
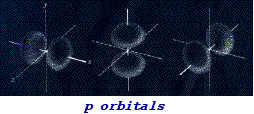
![]() s and p orbitals
s and p orbitals
Orbital 1s is the orbital corresponding to the lower energetic level and it can be described, with good approximation, as a sphere containing the nucleus in the center. At the successive energetic level we find the orbital 2s also having a spherical form but with larger dimensions due to the major average distance from the nucleus. After the orbital 2s we find three orbitals of equal energy indicated as orbitals 2px, 2py, 2pz see figure.
 |
 |
In most of cases molecules are formed by reactions of other differents molecules. However, for simplicity, we consider here the formation of a molecule through the union of single atoms.
A covalent bond is formed through the superimposition of two atomic orbitals each containing a single electron. When this happens we have the formation of a molecular orbital occupied by both electrons. Each electron is now actracted by two positive nucleus and it is in a state of minor energy with respect to its state in the isolated atom (in fact, the energy needed to draw away an electron from the nucleus is higher in the molecule).
However, in order to allow the superimposition of atomic orbitals, nuclei must approach and this process require energy to contrast the repulsive force betwen the nuclei. It follows that, in order to assure the stability of the molecule, the distance between the nuclei can not exceed a certain value.
The minimum distance allowed between the two atoms is the bond length.
The energy liberated upon the formation of bond is referred to as bond energy.
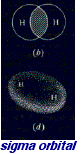
the bond existing between hydrogen atoms in the hydrogen molecule arises from the superimposition of orbitals 1s. See figure for the form of the resulting molecular orbital. Molecular orbitals having the form above are called sigma orbitals and the corresponding bonds are called sigma bonds.
The electronic configuration of fluorine is as follows:
| 1s | 2s | 2px | 2py | 2pz |
| 2 | 2 | 2 | 2 | 1 |
The superimposition of an orbital 2p (containing a single electron) of a fluorine atom with that of another fluorine atom leads to the formation of the fluorine molecule. The resulting molecular orbital can be pictured as follows:
The maximum concentration of charge is between the two fluorine atoms and the external lobes of the two p orbitals have a very small dimension. It follows that, by neglecting the external lobes, the bond F-F has the same form of that described for hydrogen and it is also a sigma bond.
![]() Methane
Methane
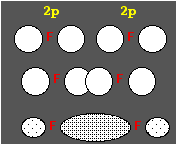
From the electron configuration of carbon
| 1s | 2s | 2px | 2py | 2pz |
| 2 | 2 | 1 | 1 |
one should expect that carbon combines with two hydrogen atoms to yield the molecule CH2. However, the most simple organic molecule is methane in which carbon combines with four hydrogen atoms (CH4). This can be explained if we assume the following "excited" electron configuration:
| 1s | 2s | 2px | 2py | 2pz |
| 2 | 1 | 1 | 1 | 1 |
 The
promotion of an electron from the orbital 2s to the orbital 2p requires energy
(about 65 kcal/mole) but the energy liberated from the formation of two additional
bonds, more than compensates for the excitation energy.
The
promotion of an electron from the orbital 2s to the orbital 2p requires energy
(about 65 kcal/mole) but the energy liberated from the formation of two additional
bonds, more than compensates for the excitation energy.
According to the configuration above we should expect that the four bonds existing in methane are not equivalent for they arise from the combination of differents kind of atomic orbitals. However, from experimental studies, is known that the four bonds are perfectly equivalents. To take in account of such experimental evidence, we need to introduce a new assumption, i.e., the existence of hybrid orbitals. These orbitals arise from the combination of orbitals s and p to yield four equivalent orbitals, called orbitals sp3, which are spacially directed from the carbon atom towards the corners of a regular tetrahedron (at an angle of 109,5°):
As expected, from its electron configuration, nitrogen combines with three hydrogen atoms to yield ammonia (NH3).
| 1s | 2s | 2px | 2py | 2pz |
| 2 | 2 | 1 | 1 | 1 |
 However,
as in the case of carbon an hybridation sp3 exists and the four resulting
orbitals are spacially directed from the nitrogen atom towards the corners of
a regular tetrahedron. Three of these orbitals are involved in the bonds with
the three hydrogen atoms while the fourth orbital is occupied by a couple of
electrons. However, the angle bond is only of 107° (not 109,5° as in
methane) and this can be explained by assuming that the volume occupied by the
isolated electron pair is larger than that occupied by hydrogen atom thus depressing
the ideal angle bond (109,5°).
However,
as in the case of carbon an hybridation sp3 exists and the four resulting
orbitals are spacially directed from the nitrogen atom towards the corners of
a regular tetrahedron. Three of these orbitals are involved in the bonds with
the three hydrogen atoms while the fourth orbital is occupied by a couple of
electrons. However, the angle bond is only of 107° (not 109,5° as in
methane) and this can be explained by assuming that the volume occupied by the
isolated electron pair is larger than that occupied by hydrogen atom thus depressing
the ideal angle bond (109,5°).
The orbital containing the isolated electron pair can bind atoms/molecules lacking electrons. For example, hydrogen ion can bind ammonia to give the ammonium ion (NH4+).
Also in water there exists an sp3 hybridization but oxygen can bound only two hydrogen atom. Therefore, two orbitals are associated with hydrogen atoms and each of the two remaining orbitals are occupied by a couple of electrons.
| 1s | 2s | 2px | 2py | 2pz |
| 2 | 2 | 2 | 1 | 1 |

Because of the two lone electron pair the angle bond results to be 105°.
Alkenes are compounds characterized by the presence of one carbon-carbon double bond, the most simple alkene is ethylene (or ethene)
H2C = CH2
In compounds containing a double bond between two carbon atoms,like ethylene, a different kind of hybridization exists. The hybrids arise from the combination of one orbital s and two orbitals p (sp2 orbitals, sp2 hybridization).
| 1s | 2s | 2p | 2p | 2p |
| 2 | 1 | 1 | 1 | 1 |
|
|
These three equivalent orbitals lie on the same plane at an angle of 120°. The term trigonal hybrids (trigonal hybridization) is used to describe these orbitals. Only two orbitals p have been used for the hybridization and the remaining orbital p is arranged perpendicularly to the plane occupied by hybrid orbitals. In ethylene, each carbon atoms bound two hydrogen atoms using two orbitals sp2 (sigma bounds), the remaining sp2 orbitals and p orbitals hold together the carbon atoms. The bond resulting from the superimposition of the two p orbitals is referred as p bond. The maximum superimposition of p orbitals is achieved when all the atoms lie on the same plane, in other words ethylene has a planar structure. |
![]() Acetylene
Acetylene
Alkines are compounds characterized by the presence of a triple bond between two carbon atoms. The most simple alkines is acetylene:
C2H2
In the case of a triple bond between carbon atoms there is the formation of two equivalents orbitals from the hybridization of one orbital p and one orbital s
| 1s | 2s | 2p | 2p | 2p |
| 2 | 1 | 1 | 1 | 1 |
the two orbitals can be schematized as follows:
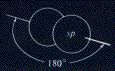
In acetylene two carbon atoms with hybridization sp exists, the four sp orbitals are used to bind together the two carbon atoms and two hydrogen atoms through sigma bonds. The resulting structure is a linear molecule in which the four atoms lie on a straight line:
H --- C ---- C ---- H
the structure of ethylene is complete when the two remaining p orbitals (each containing one electron) of one carbon atom are superimposed to the two remaining p orbitals of the other carbon atom. The formation of the two p bonds result in a cylindrical structure:
