The most simple situation in chemical bonding is the one in which a pair of electrons bound only two atoms. The bond is usually represented by a pair of dots (the electrons) or by a line. Taking hydrogen molecule as an example:
H . . H or H - H
However there are many cases in which more atoms are held togheter by one pair of electrons. Such kind of bonding is said delocalized. The most simple molecule were delocalization occurs is H3+, a molecule which is formed in electrical discharges through hydrogen gas. In this molecule there are only two electrons which bond three hydrogen atoms and it can be predicted that identical forces hold all pair of atoms together. The notation (resonance notation) used by chemist to represent the bonding in H3+ is reported here.
Please note:
No one of the structures (said canonical structures or forms) above really exist.
It is not the case that some molecule have a canonical form and some another.
Molecules do not rapidly oscillate between canonical forms.
All molecules have the same structure.
In other words, because just one structure cannot represent the real bonding situation, in which one pair electrons visits more than two nuclei, we have to draw more structures to represent the real electrons distribution. Normally, is said that the real structure is a resonance hybrid of canonical forms.
The concept of resonance, despite of its artificial nature, can help to understand the different stability of molecules. Here we list some essential rules to deal with resonance:
Resonance can occur when a molecule can be represented by several structures differing only for the distribution of electrons.
The existence of resonance (electrons delocalization) in a molecule lead to an increase of its stability. In other words, the structure of the resonance hybrid has a lower energy of the individual canonical forms we can draw. The difference in energy is said energy resonance.
Resonance is effective when the structures of canonical forms have nearly equivalent stability. The greater the number of these forms, the greater the resonance energy, i.e. the stability of the molecule.
Forms involving charge separation are less stable than uncharged ones. Very unfavorable cases are forms having more than two formal charges or having the same kind of charge on adiacent atoms. Favorable cases are those in which the negative charge is located on an atom of high electronegativity and the positive charge on an atom of low electronegativity.
As an example, let's consider the dissociation of ethanol (CH3CH2OH = CH3CH2O- + H+) and acetic acid (CH3COOH = CH3COO- + H+).
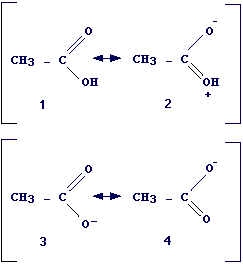
By analyzing the structure of reagents and products in the dissociation reactions above we can deduce that:
i) both the alcohol and its anion are well described by a single structure, i.e., no resonance occurs
ii) both acetic acid and acetate ion can be described by means of two canonic forms (see picture). The resonance in acetic acid involves two forms with very different stability (the form with charge separation is very instable) and then the resonance can be neglected (rule 3 and 4). On the contrary, the canonical forms for acetate are equivalents and resonance is important. Thus the difference of stability between products and reagents is larger in the case of acetic acid than in the case of the alcohol and this can explain the fact that carboxylic acids are stronger acids than alcohols.